Keywords
Abstract
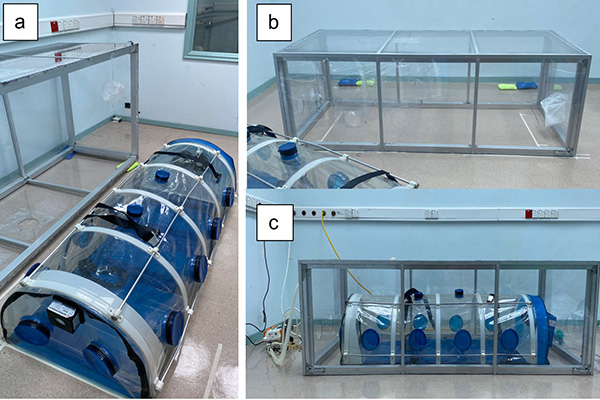
Introduction: The BIOBASE biological isolation chamber (BBIC) was used to limit the spread of SARS-CoV-2 transmission during transport of COVID-19 patients. We aim to study the effectiveness of BBIC in limiting the spread of aerosol during static transport amongst healthcare workers.
Methods: Nebulised saline 0.9% was generated to saturate aerosolised particles within the BBIC placed within a constructed outer enclosure. Negative pressure was activated and particulate matter (PM), PM10 and PM2.5 concentrations were measured over 60 minutes using sensors placed inside (Cin) and outside (Cout) the BBIC. Control, closed ports, and open port models were developed to assess the effectiveness of the BBIC in containing and evacuating aerosolised particles. The ratio of measured Cin to Cout, designated as Fiso, (Fiso = Cin/ Cout) was derived.
Results: The differences in Fiso value of PM10 compared to PM2.5 in the closed-ports test were significant at minute 15 and 25 (p < 0.001, respectively). The differences in Fiso value of PM10 compared to PM2.5 in the open-ports test were significant at minute 15 (p < 0.001), suggesting that both the closed- and open-ports tests effectively contained the PM10 compared to PM2.5 aerosolised particles. The Fiso negatively correlated with time for the open-ports (r = -0.79, p = 0.035) and closed-ports tests (r = -0.79, p = 0.035) for PM10.
Conclusions: The closed and open BBIC ports effectively contain and evacuate PM10 aerosolised particles during simulation of static transport of COVID-19 patients. The BBIC contains and evacuates PM10 more effectively than PM2.5 aerosolised particles.
References
Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199-1207. https://doi.org/10.1056/nejmoa2001316
Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4):e35797. https://doi.org/10.1371/journal.pone.0035797
Gómez-Ochoa SA, Franco OH, Rojas LZ, et al. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes [published correction appears in Am J Epidemiol. 2021 Jan 4;190(1):187]. Am J Epidemiol. 2021;190(1):161-175. https://doi.org/10.1093/aje/kwaa191
Kursumovic E, Lennane S, Cook TM. Deaths in healthcare workers due to COVID-19: the need for robust data and analysis. Anaesthesia. 2020;75(8):989-992. https://doi.org/10.1111/anae.15116
Astuti I, Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab Syndr. 2020;14(4):407-412. https://doi.org/10.1016/j.dsx.2020.04.020
Duguid JP. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei.J Hyg (Lond). 1946;44(6):471-479. https://doi.org/10.1017/s0022172400019288
Atkinson J, Chartier Y, Pessoa-Silva CL, et al., editors. Natural Ventilation for Infection Control in Health-Care Settings. Geneva: World Health Organization; 2009.
Bourouiba L. Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID -19. JAMA. 2020;323(18):1837–1838. https://doi.org/10.1001/jama.2020.4756
Morawska L, Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ Int. 2020;139:105730. https://doi.org/10.1016/j.envint.2020.105730
Booth TF, Kournikakis B, Bastien N, et al. Detection of Airborne Severe Acute Respiratory Syndrome (SARS) Coronavirus and Environmental Contamination in SARS Outbreak Units. The JID. 2025;191:1472–1477. https://doi.org/10.1086/429634
Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 582, 557–560 (2020). https://doi.org/10.1038/s41586-020-2271-3
Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26(7):1583-1591. https://doi.org/10.3201/eid2607.200885
Birgand G, Peiffer-Smadja N, Fournier S, Kerneis S, Lescure F, Lucet J. Assessment of Air Contamination by SARS-CoV-2 in Hospital Settings. JAMA Netw Open. 2020;3(12):e2033232. https://doi.org/10.1001/jamanetworkopen.2020.33232
Lindsley WG, Pearce TA, Hudnall JB, et al. Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J Occup Environ Hyg. 2012;9(7):443-449. https://doi.org/10.1080/15459624.2012.684582
World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. Sci Brief (2020).
Lindsley WG, Noti JD, Blachere FM, et al. Viable Influenza A Virus in Airborne Particles from Human Coughs, Journal of Occupational and Environmental Hygiene. 2025;12(2):107-113. https://doi.org/10.1080/15459624.2014.973113
Lee J, Yoo D, Ryu S, et al. Quantity, size distribution, and characteristics of cough-generated aerosol produced by patients with an upper respiratory tract infection. Aerosol Air Qual. Res. 2019;19:840-853. https://doi.org/10.4209/aaqr.2018.01.0031
Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air. 2006;16(5):335-347. https://doi.org/10.37934/cfdl.17.2.115135
Xie X, Li Y, Chwang A, Ho P, Seto W. How far droplets can move in indoor environments? revisiting the Wells evaporation falling curve. Indoor Air. 2007;17(3):211-225. https://doi.org/10.1111/j.1600-0668.2007.00469.x
Gralton J, Tovey E, McLaws ML, Rawlinson WD. The role of particle size in aerosolised pathogen transmission: a review. J Infect. 2011;62(1):1-13. http://dx.doi.org/10.1016/j.jinf.2010.11.010
Wei J, Li Y. Airborne spread of infectious agents in the indoor environment. Am J Infect Control. 2016;44(9 Suppl):S102-S108. https://doi.org/10.1016/j.ajic.2016.06.003
Le HD, Novak GA, Janek KC, et al. A novel box for aerosol and droplet guarding and evacuation in respiratory infection (BADGER) for COVID-19 and future outbreaks. Sci Rep. 11, 3179 (2021). https://doi.org/10.1038/s41598-021-82675-623. www.biobase.cc/Biological-Isolation-Chamber-pd46826995.html
Tsai SH, Tsang CM, Wu HR, et al. Transporting patient with suspected SARS. Emerg Infect Dis. 2004;10(7):1325-1326. https://doi.org/10.3201/eid1007.030608
Kim SC, Kong SY, Park GJ, et al. Effectiveness of negative pressure isolation stretcher and rooms for SARS-CoV-2 nosocomial infection control and maintenance of South Korean emergency department capacity. Am J Emerg Med. 2021;45:483-489. https://doi.org/10.1016/j.ajem.2020.09.081
Dindart JM, Peyrouset O, Palich R, et al. Aerial medical evacuation of health workers with suspected Ebola virus disease in Guinea Conakry-interest of a negative pressure isolation pod-a case series. BMC Emerg Med. 17, 9 (2016). https://doi.org/10.1186/s12873-017-0121-x
Begley JL, Lavery KE, Nickson CP, Brewster DJ. The aerosol box for intubation in coronavirus disease 2019 patients: an in-situ simulation crossover study. Anaesthesia. 2020;75(8):1014-1021. https://doi.org/10.1111/anae.15115
Fidler RL, Niedek CR, Teng JJ, et al. Aerosol Retention Characteristics of Barrier Devices. Anesthesiology. 2021;134(1):61-71. https://doi.org/10.1097/aln.0000000000003597
Lang AL, Shaw KM, Lozano R, Wang J. Effectiveness of a negative-pressure patient isolation hood shown using particle count. Br J Anaesth. 2020 Sep;125(3):e295-e296. https://doi.org/10.1016/j.bja.2020.05.002
Owen MK, Ensor DS, Sparks LE. Airborne particle sizes and sources found in indoor air. Atmos Environ Gen Top. 1992;26:2149–2162.
Cubillos J, Querney J, Rankin A, Moore J, Armstrong K. A multipurpose portable negative air flow isolation chamber for aerosol-generating procedures during the COVID-19 pandemic. Br J Anaesth. 2020 Jul;125(1):e179-e181 https://doi.org/10.1016/j.bja.2020.04.059
Ramdan MI, Abd Rahim I, Nik Ab Rahman NH, et al. Development Of an Affordable Negative-Pressure Full-Body Isolation Pod for Covid-19 Patient Transportation. Journal of Advanced Research in Fluid Mechanics and Thermal Sciences. 2021;88(3):137-144. https://doi.org/10.37934/arfmts.88.3.137144
Blood TC Jr, Perkins JN, Wistermayer PR, et al. COVID-19 Airway Management Isolation Chamber. Otolaryngol Head Neck Surg. 2021 Jan;164(1):74-81. https://doi.org/10.1177/0194599820942500
Lu J, Gu J, Li K, Xu C, Su W, Lai Z, Zhou D, Yu C, Xu B, Yang Z. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020; 26: 1628–31. https://doi.org/10.3201/eid2607.200764
National Academies of Sciences, Engineering, and Medicine. Rapid Expert Consultation on the Possibility of Bioaerosol Spread of SARS–CoV-2 for the COVID-19 Pandemic (April 1, 2020). 2020, Washington, DC: National Academies Press.
Morawska L, Johnson GR, Ristovski ZD, et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. Journal of Aerosol Science. 2009;40(3):256-269. https://doi.org/10.1016/j.jaerosci.2008.11.002.
Li Y, Huang X, Yu IT, Wong TW, Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2005 Apr;15(2):83-95. https://doi.org/10.1111/j.1600-0668.2004.00317.x